The basics of intestinal permeation
What is poor intestinal permeability?
Developing a formulation for the oral route is a long and complex journey in which the API (Active Pharmaceutical Ingredient) characteristics are of an utmost importance. Amidon et al. were the first, in 1995, to suggest a classification system for APIs, in which they correlated in vitro drug dissolution and in vitro permeability to in vivo bioavailability. This concept is called the Biopharmaceutical Classification System (BCS).
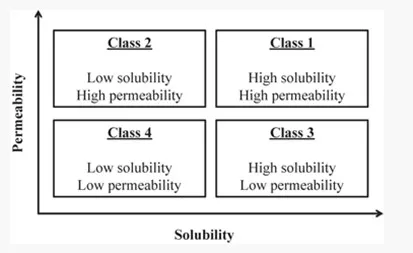
A drug is considered highly soluble when its highest therapeutic dose is soluble in < 250 mL of aqueous solution from pH 1 to 7.5.
As for the permeability, defined as how easily the API passes through biological membranes, it is considered high when > 85% of the highest therapeutic dose is absorbed on Caco-2 cells in-vitro tests. Poor permeability is a limitation of a growing number of both small molecules (BCS III and IV) and large molecules (peptides and proteins).
How does intestinal permeability work?
The intestinal epithelium has two main yet opposite functions. On one hand it prevents xenobiotics to cross the membrane and on the other hand it enables the absorption of nutrients. This dual role is safeguarded via sophisticated systems.
To facilitate absorption, several pathways are possible. The transcellular route consists in the transport of substances across a cell, either via passive diffusion (for small, non-polar molecules) or via active transport using transporters and cell energy (ATP). On the other hand, the paracellular route, mainly used by large hydrophilic solutes, works by the opening of the tight junctions, which are proteins binding the epithelial cells together.

What is an intestinal permeation enhancer?
Intestinal permeation enhancers are compounds that boost the passage of molecules through the intestinal epithelial, either via acting on the paracellular pathway, the transcellular pathway or both. Medium chain fatty acids, whose carbon chain contains 8 or 10 carbon atoms (commonly called C8-C10) are known to enhance the permeation, mainly by transiently opening the tight junctions.
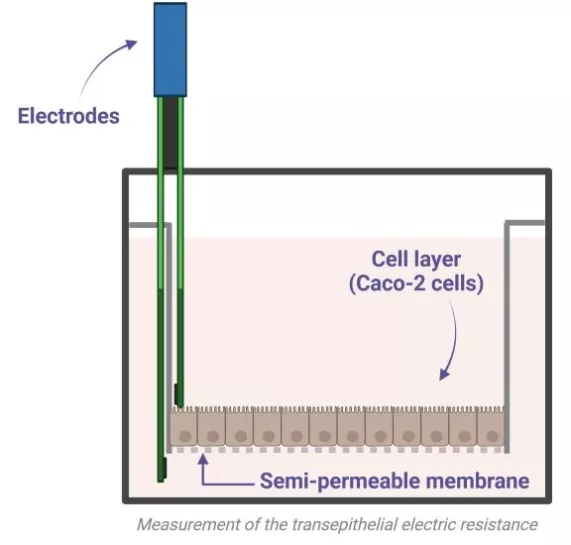
To measure the permeability enhancement properties, several methods exist. The classic set-up uses Ussing chambers, consisting of two compartments separated by a membrane (Caco-2 cells for in vitro tests, excised intestinal tissue for ex vivo tests). Each compartment is either in contact with the apical side or the basolateral side of the intestinal epithelium, and contains electrodes to apply an electrical current. The variation in electrical resistance between both sides of the membrane is followed over time: the lower the resistance is, the more the intestinal barrier is permeable.
Gattefossé acknowledged intestinal permeation enhancers
Labrasol® ALF, Labrafac™ MC60 and Capryol® 90 are all esters of C8-C10 fatty acids. The main difference consists in the alcohol part of the esters: PEG for Labrasol® ALF, glycerol for Labrafac™ MC60 and propylene glycol for Capryol® 90. They are all listed in the FDA Inactive Ingredients Database (IID). It is worth mentioning that Labrasol® ALF, Labrafac™ MC60 and Capryol® 90 act both as solubilizers and permeation enhancers, and hence contribute to an increase in oral bioavailability. By increasing solubility and intestinal permeability, they enable to formulate DCS class IIa, IIb, III and IV molecules.
| Excipient name | Chemical composition | HLB | Other functionalities |
|---|---|---|---|
| Labrasol® ALF |
Caprylocaproyl macrogol-8 glycerides EP Caprylocaproyl polyoxyl-8 glycerides NF |
12 (water-dispersible surfactant) | Solubilizers and bioavailability enhancers |
| Labrafac™ MC60 |
Glycerol monocaprylocaprate (type I) EP Glyceryl mono and dicaprylocaprate NF |
6 (water-insoluble surfactant) | |
| Capryol® 90 |
Propylene glycol monocaprylate (type II) EP Propylene glycol monocaprylate NF |
5 (water-insoluble surfactant) |
What about large molecules?
Oral delivery of biologics has become a common problematic in nowadays formulation projects. One of the key hurdles that need to be addressed is the passage through the intestinal barrier, as biologics are large molecules that can not diffuse passively through the cell layer. Gattefossé intestinal permeation enhancers demonstrated their ability to enhance large molecules permeability in a safe and efficient manner in several studies, using insulin as a model (McCartney et al., 2019; McCartney et al., 2023; Ukai et al., 2020b). As for the threshold of molecular weight critical for permeability, it has been demonstrated in vitro that molecules up to 40 kDa can pass through tight junctions safely.
Excipients for solubility and bioavailability enhancement
Poor solubility, poor permeability, and pre-systemic elimination are factors that can limit absorption of some drugs. Lipid excipients have the capability to overcome these hurdles and enhance oral bioavailability through different mechanisms.
Latest outcomes from ex vivo and in vivo studies with Labrasol® ALF and Labrafac™ MC60, intestinal permeation enhancers
Presentation of the latest results of ex vivo and in vivo studies with Labrasol® ALF and Labrafac™ MC60, intestinal permeation enhancers.Speaker: Dr Fiona McCartney, University College Dublin
