A nonionic water-insoluble surfactant used as cosurfactant in oral lipid-based formulation SEDDS and SMEDDS. Cosurfactant and solubilizer in topical dosage forms.
Names & synonyms
| USP NF Name | Propylene Glycol Monocaprylate NF |
|---|---|
| EP Name | / |
| UNII Code (FDA) | RT9P9S09QI |
| Preferred Substance Name (FDA) | PROPYLENE GLYCOL CAPRYLATE |
| Handbook of Pharmaceutical Excipients | Propylene glycol monocaprylate |
| Chemical description | Consists of propylene glycol esters of caprylic acid (C8), mainly composed of monoesters and a small fraction of diesters. |
Key characteristics
| Product form | Oily liquid |
|---|---|
| Viscosity (mPa.s) | 20 (20°C) |
| HLB | 5 |

Main functionalities
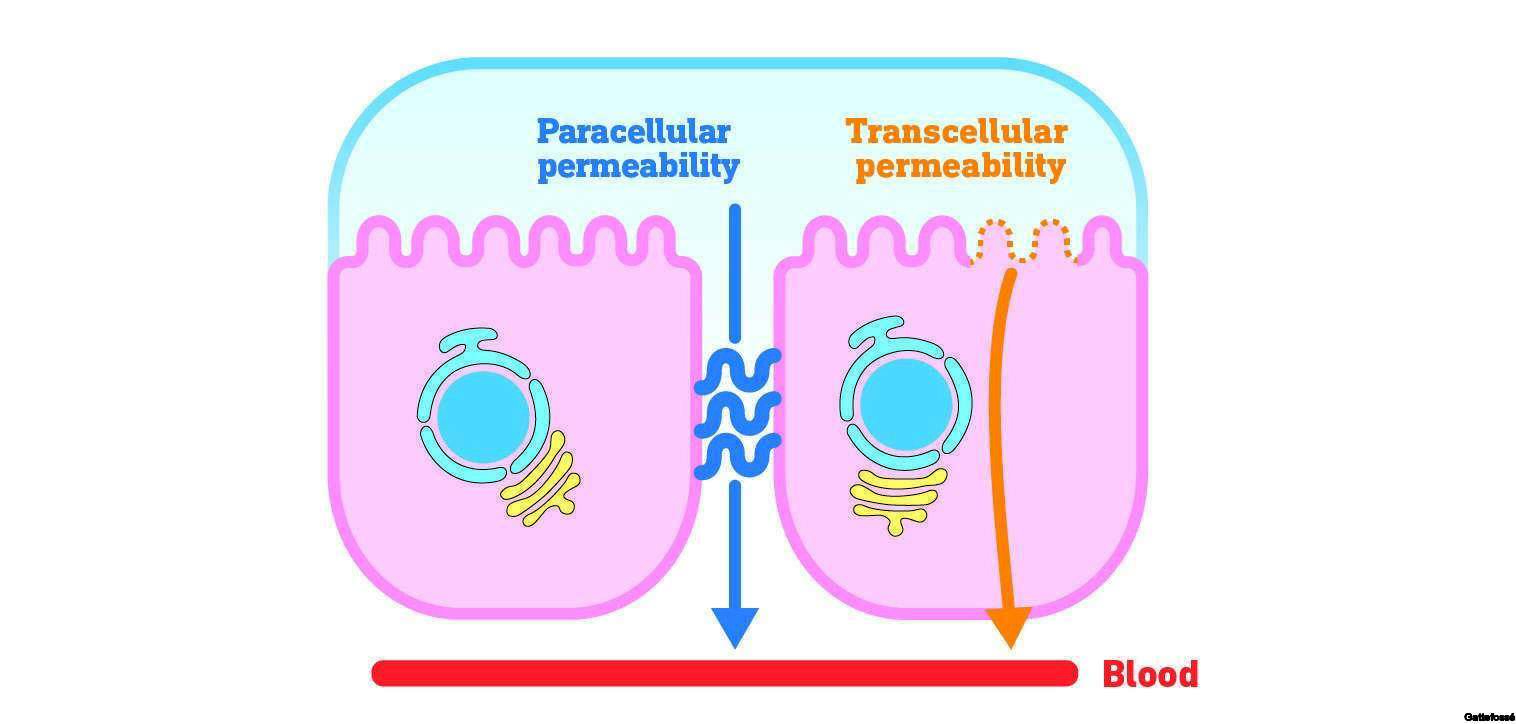
Solubilizer for poorly-soluble APIs and bioavailability enhancer. Co-surfactant in LFCS Type II (SEDDS) and Type III (SMEDDS) formulations.
Solubilizer and skin penetration enhancer. Co-surfactant for microemulsion in topical formulation.
Safety of use is inferred by precedence of use in approved pharmaceutical products.
Main formulation technologies
- Oral applications: self-emulsifying lipid formulation
- Topical emulgel, microemulsion
Resources
Technical documentation
Brochures
-
Gattefosse brochure Selecting the best excipients to optimize drug delivery
-
Gattefosse brochure Lipid excipients for cannabinoid drug products
-
Gattefosse brochure Oral drug delivery with lipid excipients
-
Brochure Gattefosse Lipid-based formulations Bio-enhancers by nature
-
Gattefosse brochure topical drug delivery with Lipid excipients
-
Gattefosse Brochure Skin-Friendly Textures
-
Gattefosse brochure veterinary medicines with Lipid excipients
Scientific document
-
Poster Pharmsci 2019: In vitro evaluation of leuprolide-containing solid lipid-based nanosuspensions
-
Poster AAPS 2013: Self formulation protocol I Solubiity determination in liquid and solid excipient
-
Poster AAPS 2013: SELF formulation protocol II Excipient selection diagram design and selection of prototype formulations
-
Poster AAPS 2013: SELF formulation protocol III Impact of lipolysis on prototype formulations
-
Poster AAPS 2014: Establishment of a Binary Phase Diagrams Database for the Development of Self Emulsifying Lipid Based Formulations
-
Poster CRS 2019: In Vitro Studies Indicate Promising Properties Of Solid Lipid Nanosuspensions for oral peptide delivery
-
Poster CRS 2024 - Rheology as a tool for characterizing topical product formulations: impact of solubilizer variation on stability and structure
Associated content
Excipients for solubility and bioavailability enhancement
Poor solubility, poor permeability, and pre-systemic elimination are factors that…
Selecting penetration enhancers, for transdermal delivery
To overcome the skin barrier properties, many strategies exist, among which the use…