PIT deviation for fast and accurate measure of HLB
HLB (hydrophilic lipophilic balance) is a critical parameter used to characterize surfactants.
The historical practical method still in use has major drawbacks: operator dependent as it relies on a visual assessment, room temperature dependent, not accurate, as values are given ±1 and time consuming: at least 1 day for 1 HLB measurement.
A new method (PIT-deviation) has been developed in our R&D laboratory to overcome these drawbacks.
A new method to gain time and accuracy
It relies on the concept of Phase Inversion Temperature (PIT) introduced by Shinoda et al. in 1967. The continuous phase of a direct emulsion is water, whereas it is oil for reverse emulsion. As conductivity is nil in oil and not in water, a sudden drop in conductivity indicates an inversion of phase of the emulsion.
For practical purpose, NaCl brine is used as aqueous phase to have higher values of conductivity and n-octane as oily phase to enable measures around room temperature avoiding crystallization and evaporation. While increasing the temperature, the conformation of the ethoxylated surfactant changes (change in packing parameter), leading to an inversion of the emulsion which can be evaluated by the drop in conductivity. This is the Phase Inversion Temperature.
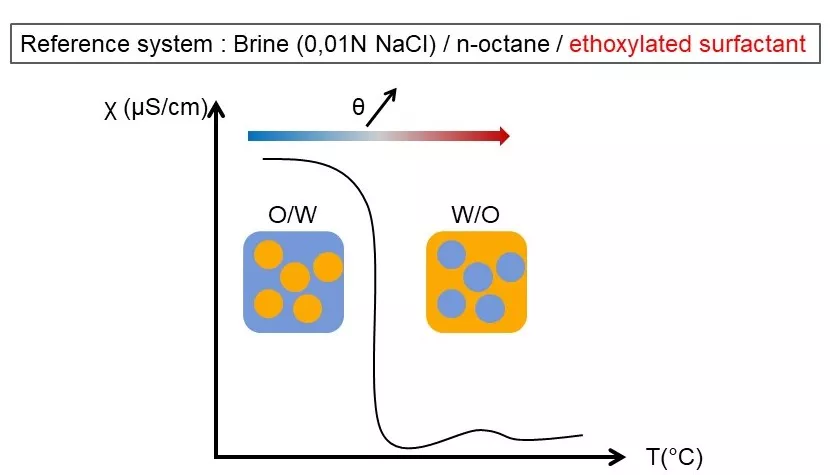
When introducing a second surfactant to the system, it settles at the interface of the emulsion next to the first one and the global packing parameter changes, hence the emulsion organization. This induces a deviation in the PIT value which is proportional to the mass fraction of the surfactant. Depending on the nature of the surfactant the slope can be positive (eg Sodium lauryl sulfate), nil (eg Tefose® 1500) or negative (eg Emullium® mellifera).

Using a range of surfactants, a correlation (r²=0.99) could be established between the HLB using the conventional method and the new PIT-deviation method.

The main advantages of this PIT-deviation method are:
- Automated method with the use of an in-house designed software
- Rapidity: 3 hours (vs 1 day for conventional method)
- Accuracy of the method as it is non-operator dependent
A (generous) slice of scientific background
Surfactants are compounds that lower the surface tension (or interfacial tension) between two liquids. They are constituted of an hydrophilic part and an hydrophobic part.
Surfactants with strong affinity for water form a direct emulsion (oil-in-water, O/W), whereas surfactants with strong affinity for oil form a reverse emulsion (water in oil, W/O).
The surfactant spatial conformation depends on the chemistry of both parts and is characterized by the packing parameter. Note that the packing parameter is not a constant and is affected by the temperature, the solvent, the ionic strength, etc.
The spatial conformation (i.e. the packing parameter, PP) of the surfactant at the interface of the two liquids orientates the emulsion organization:
- PP<1: O/W emulsion
- PP ≈ 1: bi-continuous phase
- P>1: W/O emulsion

To characterize surfactants, the concept of Hydrophilic Lipophilic Balance (HLB) was first developed in 1946 by Griffin et al. Equations were established to calculate the HLB from characteristics of the surfactant such as molar mass of the hydrophilic part, molar mass, saponification value and acid value of the acid. Davies et al. (1957) proposed an extended equation for complex surfactant, taking into account constants for each substitution group. However, both methods lead to different values and for more sophisticated surfactants the equations could not be employed.
Therefore, a practical method was developed to measure HLB using two reference surfactants for which both equations lead to similar values: Tween 80 (HLBTween80 ≈ 15) and Span 80 (HLBSpan80 ≈ 4) and knowing that the HLB of a mixture of surfactants is proportional to the mass of each surfactant:

First, a range of emulsions is prepared in standardized conditions, using 20% paraffin oil, 75% water and 5% of a mixture of Span:Tween at specific ratios (100:0, 82:18, …, 0:100). The stability of the emulsion is observed. When no or limited creaming is observed it corresponds to the HLBrequired, 10 in these conditions.

Then, the same protocol is used with an unknown surfactant to determine its HLB. If the surfactant has an affinity for water, it will be mixed with Tween 80. If it is for oil, it will be mixed with Span 80. In the case of Tefose® 1500 below, its HLB is evaluated with Tween 80.

Using the above equation and knowing the relative proportion of Tefose® 1500 and Tween 80 used for the stable emulsion C, the HLB of Tefose® 1500 can be deduced:

Although this practical method has been used for decades to determine HLB, there are some major drawbacks due to the facts that it is:
- operator dependent as it relies on a visual assessment
- room temperature dependent
- not accurate, as values are given ±1
- time consuming: at least 1 day for 1 HLB measurement