Harnessing lipids as plasticizers in hot-melt extrusion: a gateway to innovation
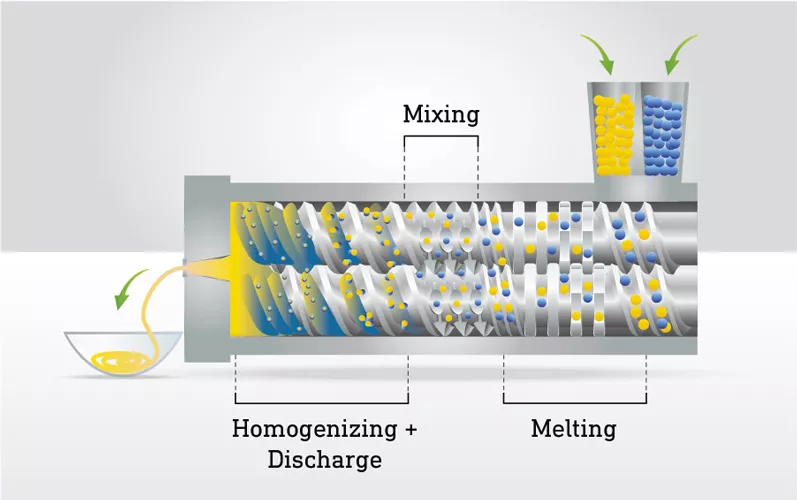
What is hot-melt extrusion?
Hot-melt extrusion (HME) is an industrial process initially created for the plastic, rubber, and food industries. However, it was revisited for the pharmaceutical industry later, using mostly polymers.
In formulation, HME is a process widely used to manufacture amorphous solid dispersions (ASD), an advantageous approach for poorly-soluble APIs. Indeed, in an ASD, the active pharmaceutical ingredient (API) is dispersed in an amorphous polymer matrix, preventing the drug from crystallizing, thus improving its dissolution rate for a better bioavailability.
Why use plasticizers in extrusion?
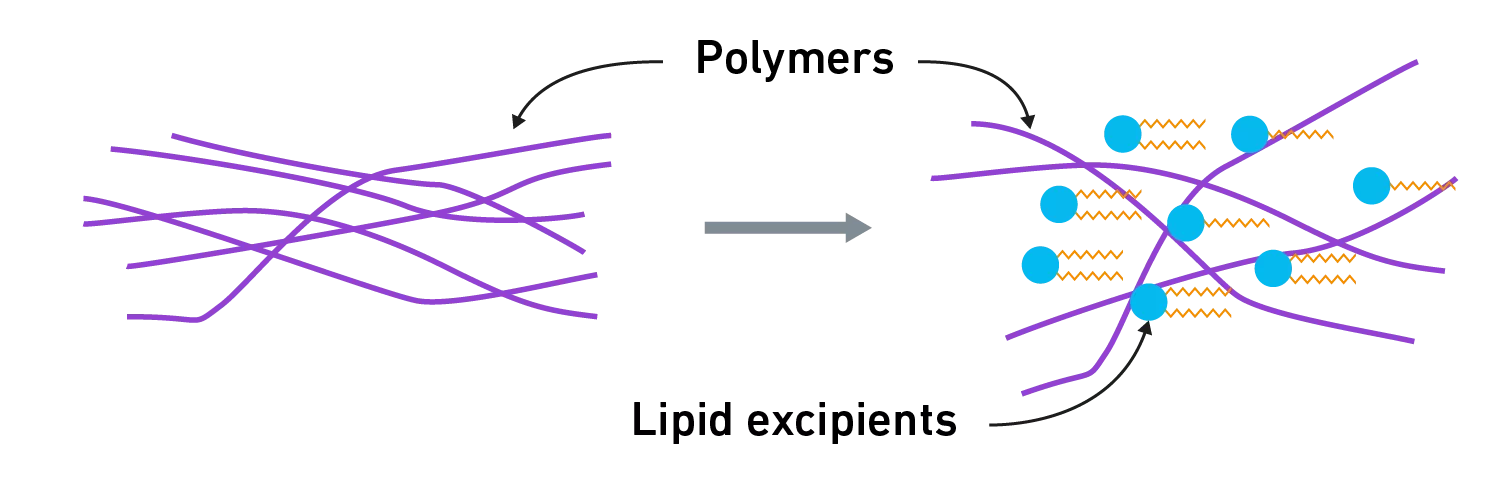
Polymers used in HME process are amorphous materials, characterized by a glass transition temperature (“Tg”), which ranges from 100 to 200°C depending on their composition. During the process, it is necessary to go beyond the Tg as it represents the point where the polymer transitions from a hard, glassy state to a flexible, rubbery state, thus acquiring the required flow and deformation to mix the API in the polymer.
Lipid excipients are demonstrated plasticizers. Due to their low molecular weight, they locate at the interspace of the entangled polymer chains and increase their mobility facilitating polymer extrusion.
- They reduce the Tg, allowing extrusion to occur at lower temperatures: this is particularly interesting for heat-sensitive APIs, as well as for the improvement of process safety.
- They reduce engine torque, thus allowing a decrease in the equipment’s energy consumption.
What other benefits bring lipid excipients to ASDs?
Improve drug release
The addition of Gelucire® 50/13 and Gelucire® 44/14 were reported to accelerate and complete drug release from hydroxypropyl methylcellulose acetate succinate based extrudates (Maniruzzaman et al., and El-Badry). The observed synergistic effects on API dissolution were due to the presence of the solid surfactant that facilitated the formation of an ASD at high drug load and improved the solubility of the drug into the polymer matrix. Our latest work with Gelucire® 48/16 and Gelucire® 50/13 added to a ticagrelor ASD showed the same tendency.
Increase stability
Keen et al. showed the addition of Compritol® 888 ATO could stabilize a polymer-based ASD produced by HME and the Kinetisol process. Due to its water insolubility, the lipid reduced the hydrophilicity of the system. This in turn decelerated both water diffusion into the dosage form and precipitation of the amorphous drug.
Re-invent dosage form production with lipid extrusion
Extrusion can also be used for modified-release, or taste-masking formulation technologies. Beyond those uses, novel dosage forms can also be developed.
Develop solid lipid nanoparticles
Patil et al. investigated the production of Compritol® 888 ATO-based solid lipid nanoparticles by combining HME with high pressure homogenization. This new approach enables continuous and scalable nanoparticle development with better process control. Nanoparticles of below 200 nm were obtained with decreased polydispersion indices and zeta potential.
Transform liquid SMEDDS into solid SMEDDS
A liquid self-microemulsifying drug delivery system (SMEDDS) consisting of Plurol® Diisostearique and Transcutol® HP was transformed into solid SMEDDS through extrusion. The solid SMEDDS enabled fast and complete release of the API at pH 6.8 whilst reducing release in acidic pH (Silva et al).
Obtain solid SMEDDS by 3D-printing
Recent publications suggested that a semi-solid lipid excipient could be formulated into self-microemulsifying tablets using an extrusion process followed by 3D printing. Vithani et al blended a poloxamer with the drug and surfactants (Gelucire® 44/14 and Gelucire® 48/16), and then printed the solid SMEDDS into different geometrical shapes. The dispersion properties of the printed dosage units were not affected by 3D printing suggesting a potential future approach to develop lipid based personalized medicines.
Discover our lipid excipients used in HME!
|
Excipient name |
Description | Form |
Melting
range (°C)
|
Main functionality |
|---|---|---|---|---|
| Capryol® 90 |
Propylene glycol monocaprylate (type II) EP Propylene glycol monocaprylate NF |
Liquid | NA | Solubility and oral bioavailability enhancement |
| Compritol® 888 ATO |
Glycerol dibehenate EP Glyceryl dibehenate NF / Ch.P. |
Powder | 65 - 77 | Sustained release, taste-masking, API protection |
| Gelucire® 44/14 |
Lauroyl macrogol-32 glycerides EP Lauroyl polyoxyl-32 glycerides NF Lauroyl macrogolglycerides (32) Ch.P. |
Block | 42.5 - 47.5 | Solubility and oral bioavailability enhancement |
| Gelucire® 48/16 |
Macrogol-32 stearate (type I) EP Polyoxyl-32 stearate (type I) NF |
Pellets | 46 - 50 | |
| Gelucire® 50/13 |
Stearoyl macrogol-32 glycerides EP Stearoyl polyoxyl-32 glycerides NF |
Pellets | 46 - 51 | |
| Mixture of lauroyl polyoxyl-32 glycerides EP / NF and PEG 6000 EP / NF | Pellets | 57 - 62 | ||
| Labrafac |
Triglycerides medium-chain EP Medium-chain triglycerides NF Medium-chain fatty acid triglycerides JPE |
Liquid | NA | |
| Labrasol® ALF |
Caprylocaproyl macrogol-8 glycerides EP Caprylocaproyl polyoxyl-8 glycerides NF |
Liquid | NA | |
| Precirol® ATO 5 |
Glycerol distearate (type I) EP Glyceryl distearate NF |
Powder | 50 - 60 | Taste-masking, sustained release |