Foam, an emerging topical dosage form
Foams are gaining popularity as pharmaceutical dosage forms as they provide scope for innovation. They offer the patient an attractive dosage form, for example for pediatric use, and for the pharmaceutical laboratories, they offer possibilities of life cycle management. From the formulator point of view, they are traditional formulations of emulsion or microemulsion, and the device is transforming them into foams.
Light or creamy, choose the texture of your foam
Foams can be produced using two distinct technologies:
-
In a propellant-free device, the formulation consists of a microemulsion. The light texture obtained is suitable for quick absorption without residue and is convenient for hair-bearing skin. It may, for example, be used with disinfectant or anti-inflammatory drugs.
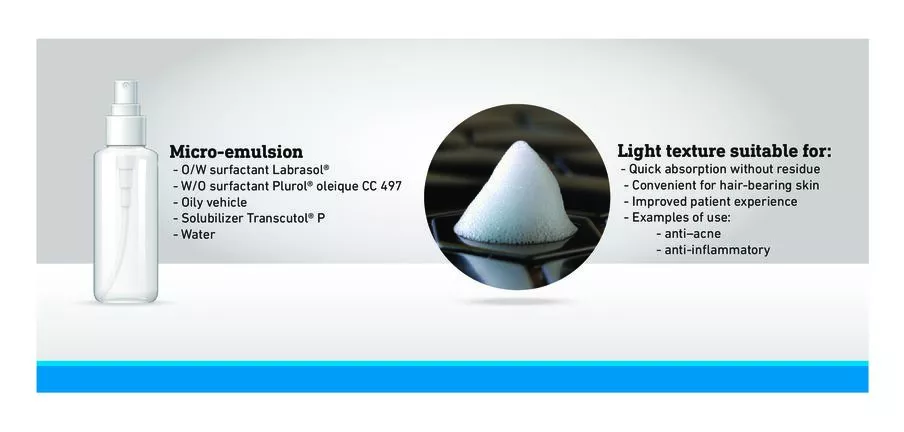
- In an aerosol using propellant gas, the formulation consists of an emulsion. The creamy foam obtained is suitable for damaged or sensitive skin (e.g., psoriasis or atopy).

Winning excipient combinations for foams
Winning trio for propellant-free foam

-
Labrasol®, liquid surfactant, good solubilizer with excellent foaming capacity
-
Plurol® Oleique CC 497, liquid co-surfactant, compulsory to get the micro-emulsion
-
Labrafac™ Lipophile WL 1349, oily vehicle with emollient properties
Winning trio for propellant foam

-
Tefose® 63, O/W emulsifier, with excellent foam capacity and high mucosal tolerance
-
Labrafil® M 1944 CS, O/W surfactant, to enhance heat stability
-
Transcutol® P, a safe and efficient solubilizer and skin penetration enhancer

Gattefossé can provide formulation tips for pharmaceutical foams using propellant and propellant-free devices, guidelines on how to formulate and select excipients, and case studies with model drugs.